Basso Giacomo [Department of Neurosciences, Rehabilitation, Ophthalmology, Genetic and Maternal Infantile Sciences (DINOGMI), University of Genova – Genova, Italy]
Bargeri Silvia [Unit of Clinical Epidemiology, IRCCS Istituto Ortopedico Galeazzi, Milan, Italy]
Ignazio Geraci [Department of Neurosciences, Rehabilitation, Ophthalmology, Genetic and Maternal Infantile Sciences (DINOGMI), University of Genova – Genova, Italy]
Greta Castellini [Unit of Clinical Epidemiology, IRCCS Istituto Ortopedico Galeazzi, Milan, Italy]
Alessandro Chiarotto [Department of Health Sciences, Faculty of Science, VU University Amsterdam, Amsterdam Movement Sciences, The Netherlands]
Silvia Gianola [Unit of Clinical Epidemiology, IRCCS Istituto Ortopedico Galeazzi, Milan, Italy]
Raymond Ostelo [Department of Health Sciences, Faculty of Science, VU University Amsterdam, Amsterdam Movement Sciences, The Netherlands]
Marco Testa [Department of Neurosciences, Rehabilitation, Ophthalmology, Genetic and Maternal Infantile Sciences (DINOGMI), University of Genova – Genova, Italy]
Tiziano Innocenti [Department of Health Sciences, Faculty of Science, VU University Amsterdam, Amsterdam Movement Sciences, The Netherlands]
Bias in the design, conduct or reporting of RCTs can result in inaccuracies in systematic reviews or guidelines and subsequent errors in clinical practice, overestimating or underestimating the effects of an intervention. A prominent issue is “reporting bias”, which may occur when there is a discrepancy between what was planned in the protocol and what is actually reported in the published manuscript of the RCTs.
Despite some improvement over time, the quality and reporting standards of trials in the exercise for chronic low back pain (CLBP), field is suboptimal. However, the extent of discrepancies between registered protocols and published manuscripts in CLBP trials remains uncertain.
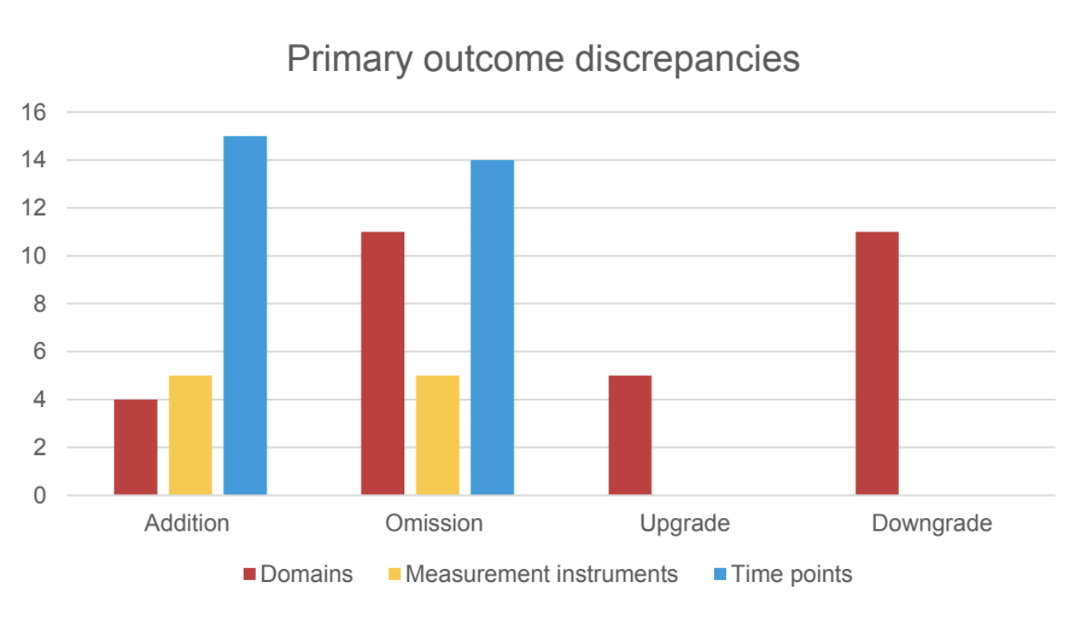
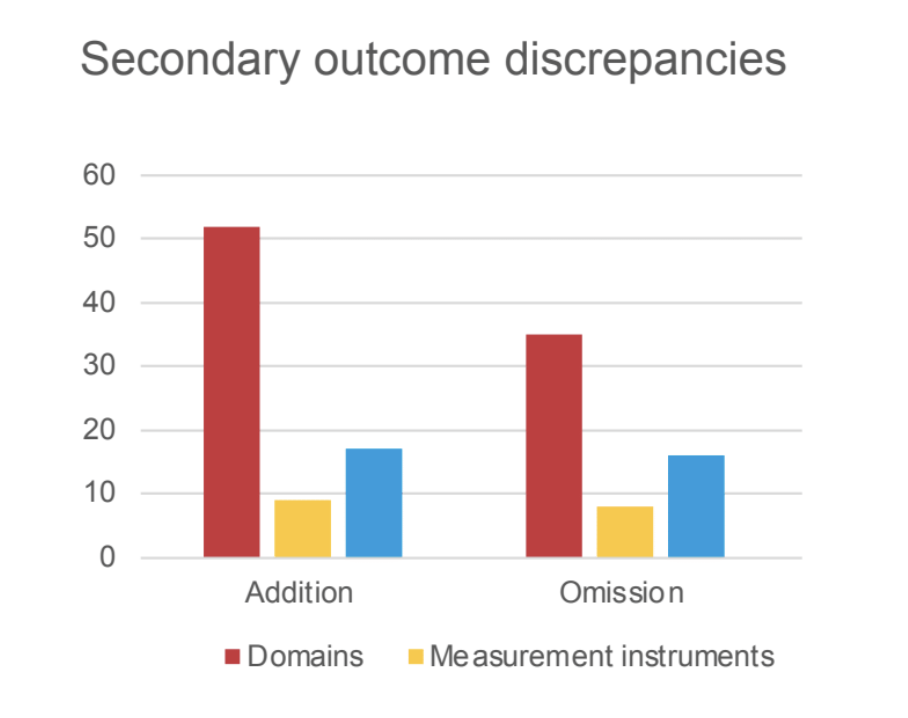
This is a meta-research study, prospectively registered. We started from the 2021 ‘Exercise therapy for chronic low back pain’ Cochrane review to select all RCTs reporting a protocol registration on a primary register of the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) or in ClinicalTrials.gov. Standardized data collection forms were developed to record information from both registered protocol and published manuscript. Independent pairs of reviewers assessed discrepancies between registered protocol and published manuscript for the reporting of primary and secondary outcomes domains, measurement instruments, time-points, number of arms and statistical analysis plans (if attached). Outcome discrepancies were characterized as addition, omission, upgrade or downgrade. We used descriptive statistics to assess the proportion of RCTs with and without a discrepancy.
We included 116 RCTs reporting an available protocol registration. Overall, 100 RCTs (86.2%) distinguished between primary and secondary outcomes. Of these, 39 RCTs (39.0%) reported one or more discrepancies in primary outcomes, and 78 RCTs (78.0%) reported one or more discrepancies in secondary outcomes. Focusing on discrepancies for the primary outcome, 64.5% of added, upgraded or downgraded outcomes favored statistically significant effects. Few RCTs (n=6) reported discrepancies in the number of arms. Statistical analysis plans were poorly reported in the registered protocols (n=3) for being compared to the publications.
We found substantial outcome discrepancies comparing registered protocols and published manuscripts in RCTs assessing exercise interventions for patients with cLBP, with some impacting the statistical significance of the effects. Readers are encouraged to approach RCTs results in this field with caution.
Hart B, Lundh A, Bero L. Effect of reporting bias on meta-analyses of drug trials: reanalysis of meta-analyses. BMJ. 2012;344:d7202.
Page MJ, Higgins JPT, Sterne JAC. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
Boutron I, Page MJ, Higgins JPT, Altman DG, Lundh A, Hróbjartsson A. Chapter 7: Considering bias and conflicts of interest among the included studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
Hayden JA, Ellis J, Ogilvie R, Boulos L, Stanojevic S. Meta-epidemiological study of publication integrity, and quality of conduct and reporting of randomized trials included in a systematic review of low back pain. J Clin Epidemiol. 2021;134:65-78.