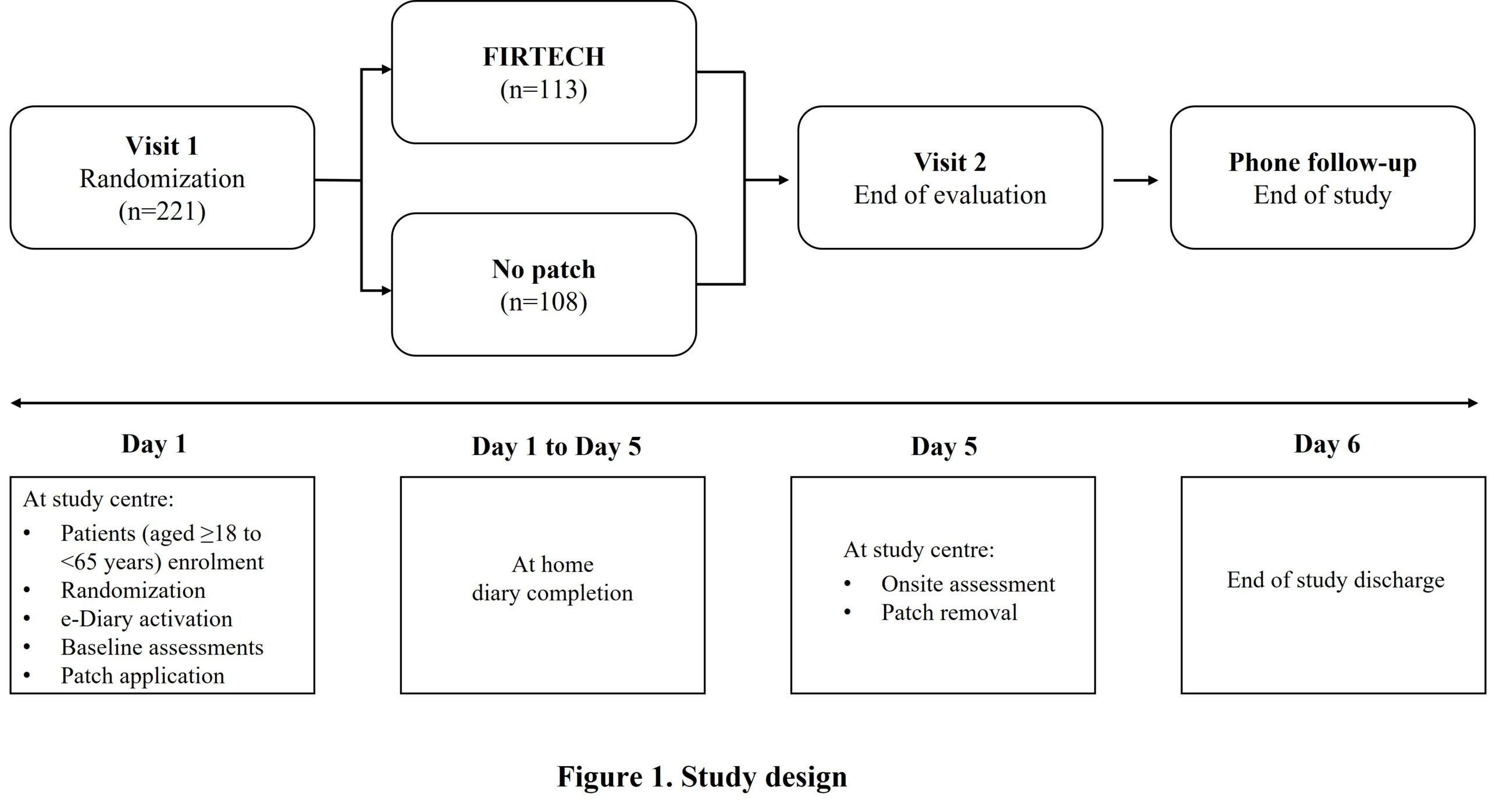
Assessment of efficacy and safety of FIRTECH patch for treatment of acute mild-to-moderate low back pain: A phase 3 randomized clinical trial
Assessment of efficacy and safety of FIRTECH patch for treatment of acute mild-to-moderate low back pain: A phase 3 randomized clinical trial
Introduction
Low back pain (LBP) has the highest prevalence among musculoskeletal conditions affecting approximately 619 million people worldwide and stands as the primary cause of disability on a global scale1. Non-pharmacological therapies are recommended as the first line of treatment for LBP2, various guidelines including Danish, US and UK support the use of exercise as monotherapy or as multimodal approach in combination with other non-pharmacological therapies3,4. FIRTECH is a non-pharmacological infrared (IR) patch containing bioceramic particles that harness the body’s natural heat and re-emit the IR energy. This energy can penetrate the skin and provide beneficial effects, such as antioxidative, anti-inflammatory, and tissue vasodilatory effects5,6. This trial investigated the efficacy and safety of FIRTECH patch in the management of acute mild-to-moderate LBP.
Methods
In this open-label trial (NCT05137041), patients with acute mild-to-moderate LBP (<1M duration, intensity ≤6 on 0-10 Numerical Rating Scale [ NRS ]) were randomized to either FIRTECH patch or no-patch control arm (Fig 1). The patch was applied on Day1 (baseline) and removed on Day5. Primary endpoint: NRS responder rate at Day 5 by no difference (between group) hypothesis using Fisher’s exact test. Secondary endpoints: Normalized Sum of Pain Intensity Difference over 5 days (SPID0-5); change from baseline to Day 5 in Roland-Morris Disability Questionnaire (RMDQ) score, mobility evaluation (Schöber’s Test), time to reach acceptable pain (TTAP), time to reach no pain (TTNP), time course of pain intensity difference (PID) and time course of pain relief; normalized sum of total pain relief over 5 days (TOTPAR0-5) and safety. Descriptive and inferential analyses were used.
Results
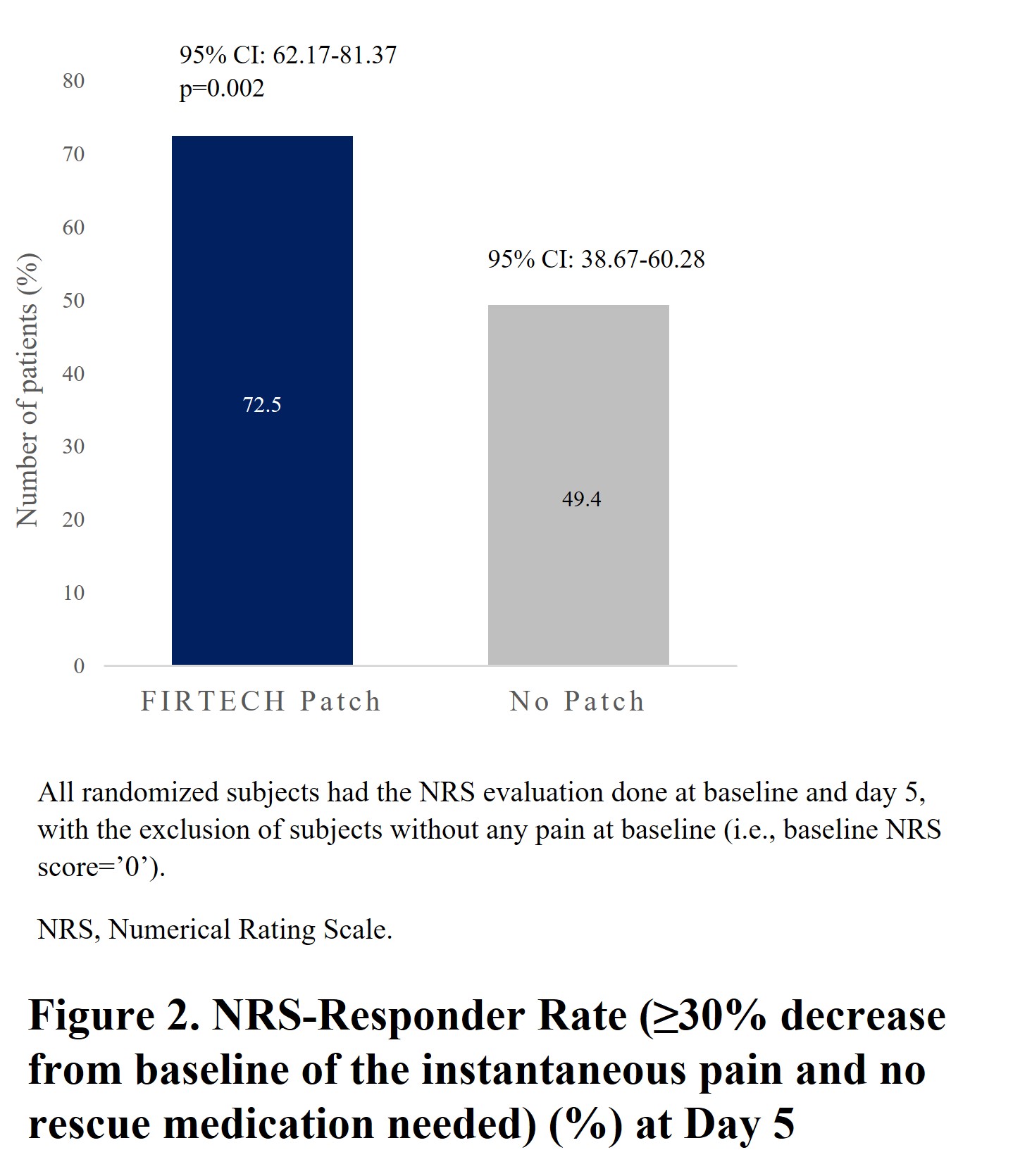
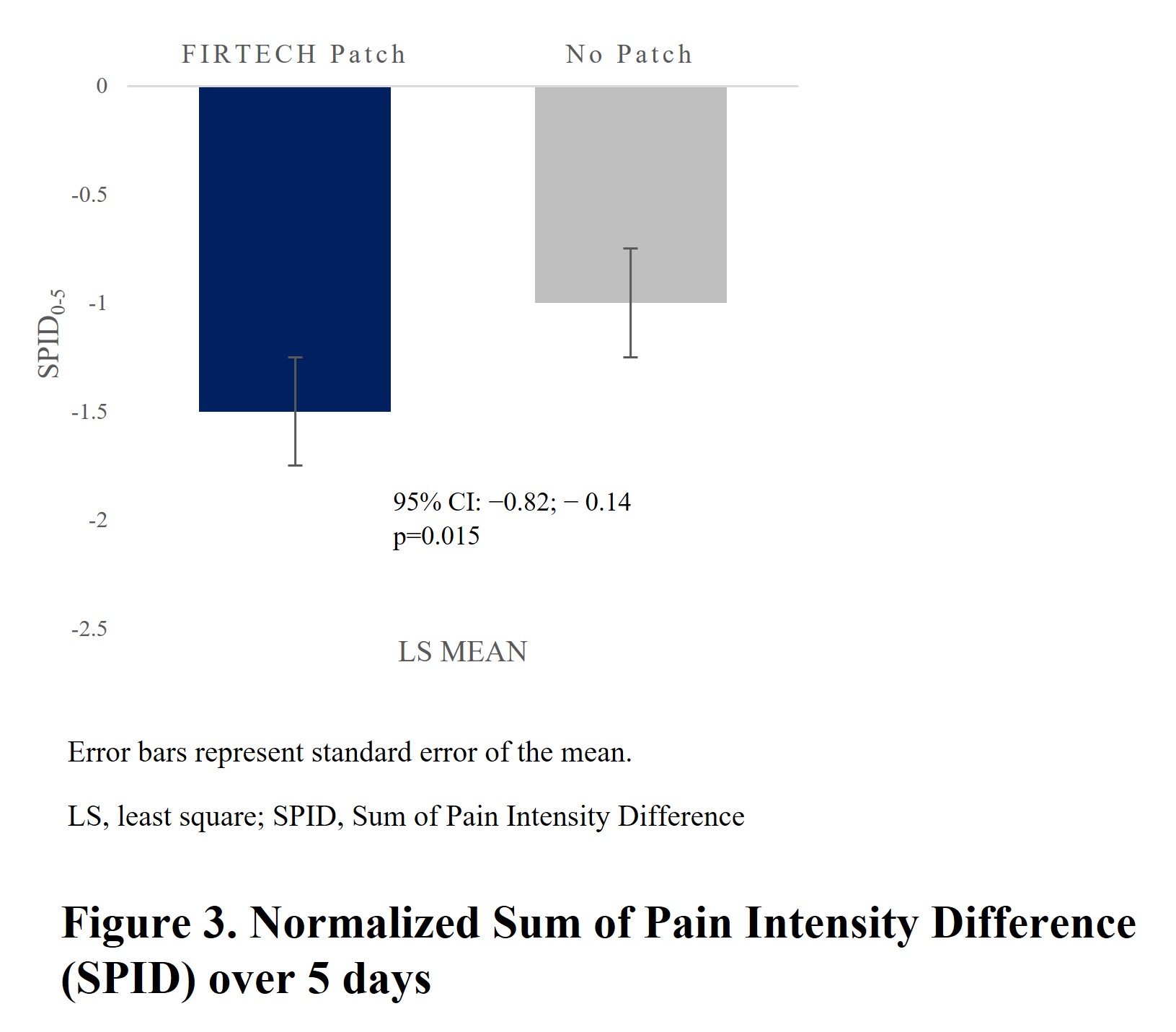
Among 221 randomized subjects (FIRTECH, n=113; no patch, n=108) of mean (SD) age 45.2 (12.97) years, 54.8% were female. NRS-responder rate was significantly higher in FIRTECH (72.5%) vs no-patch arm (49.4%; p=0.002) (Fig 2). Among non-responders, 3 (3.3%) subjects in FIRTECH and 14 (15.7%) in no-patch arms used rescue medication. The FIRTECH arm showed significant decrease in normalized SPID0-5 vs no-patch arm (LSM difference [95% CI]: −0.5 [−0.82, −1.14]; p=0.015) (Fig 3). Mobility was improved in the FIRTECH arm for Schöber’s Test (1.0 [0.511, 1.581]; p<0.001). Change in RMDQ score, TTAP and TTNP were not significant. Time course of PID, time course of pain relief and TOTPAR0-5 (LSM 1.5 vs 0.8) over 5 days demonstrated better pain relief for FIRTECH vs no-patch starting from Day1-evening. Treatment emergent adverse events were reported in 18 subjects in FIRTECH vs 7 in no-patch arm.
Discussion and Conclusion
The phase 3 trial data demonstrated promising evidence on the effectiveness of FIRTECH patch in relieving pain and improving mobility in patients with LBP up to 5 days. Additionally, the FIRTECH patch demonstrated a favourable safety profile and was well-tolerated. These IR patches can be a potential non-pharmacological option in the management of musculoskeletal pain that may help physiotherapy to achieve functional improvement and avoid chronicity of pain through rapid pain relief and restoration of patient’s ability.
Funding
This study was funded by Sanofi.
REFERENCES
- Low back pain. World Health Organization (available on Low back pain (who.int), accessed July 29, 2023).
- McSwan J, et al. J Pain Res. 2021;14:2943–2958.
- Shipton EA. Pain Ther. 2018;7:127–137.
- Mobasheri A. J Pain Res. 2022;15:3479–3482.
- Mori MM, et al. Acta Biomed. 2019;90(3):287-292.
- Nunes RFH, et al. J Hum Kinet. 2019;70:135–144.